Stores of Energy: Types, Transfer, and Applications
Energy, what is the first thing that comes to your mind when you think about this word? Is it a fire burning, or an electric shock, or Goku in Dragon Ball Z throwing an energy blast.
Now what if I tell you, that although you may think that all these are different things but they are actually the exact same thing. It is just like you wearing different clothes on different days.
Energy is one of the most fundamental concepts in physics, and it is very crucial for us to understand this concept if we want to understand how the world operates.
From the heat of the sun to the electricity powering our homes, energy exists in various forms and can be stored in different ways.
But no matter what happens: “We can never create or destroy Energy, we can only convert it from one form to another” Even Goku needed to eat a lot of food to produce his energy blasts.
Also remember, the unit of measurement for all types of energy is joules (J).
In this article, we will explore the 8 main stores of energy, their unique characteristics, and practical examples of how they are converted from one form to another.
Understanding the 8 Stores of Energy
Energy can be stored in many ways, each crucial for different processes and applications. The main stores of energy are:
Types of Energy Stores:
Chapter 1
Kinetic Energy
Whenever an object is moving, it has a form of energy, and the energy that an object has when it is moving is known as kinetic energy.

Kinetic energy depends on two factors:
1. The mass of the object : more the mass, more the KE
2. The velocity of the object: faster the object, more will be KE
The formula for kinetic energy is:
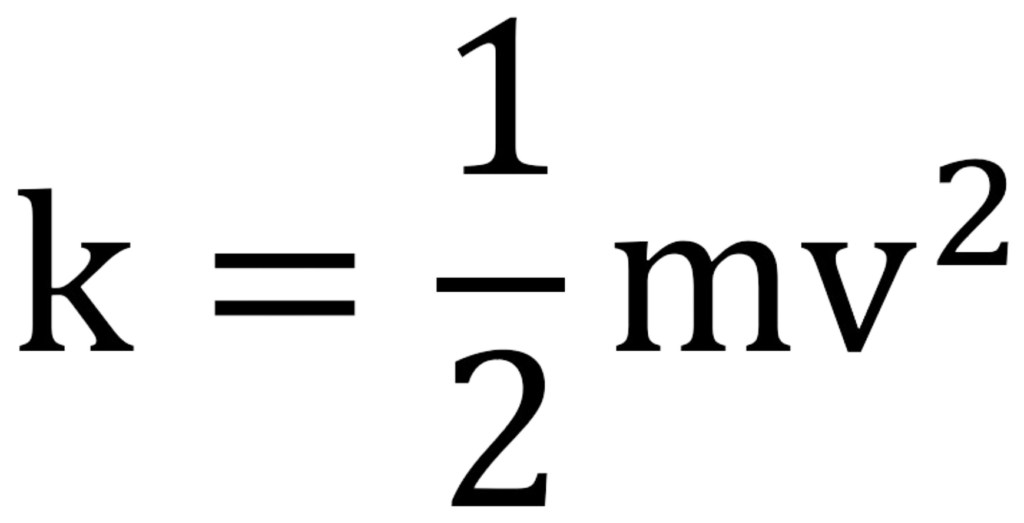
It means the faster an object moves and the heavier it is, the more kinetic energy it will carry.

Solved Example : Calculate the kinetic energy of a 60 kg boy who is running at a speed of 5 m/s.

Solution:
Given:
- Mass of the object, m=60kg
- Velocity of the object, v=5m/s
Using the formula for kinetic energy
KE = 1/2 mv2
Plugging the values
KE = 1/2 x 60kg x (5m/s)2
= 750J
Therefore, the kinetic energy of the boy is 750 joules (J)
Chapter 2
Thermal Energy (Heat)
Thermal energy, or heat energy, is the internal energy in a substance due to the movement of its particles. This energy increases as the temperature of the substance increases.

Thermal energy depends on:
1. Mass (m)
2. Temperature (T)
3. Specific Heat Capacity (C) (it is a property of a material)
The formula for calculating the change in thermal energy is:
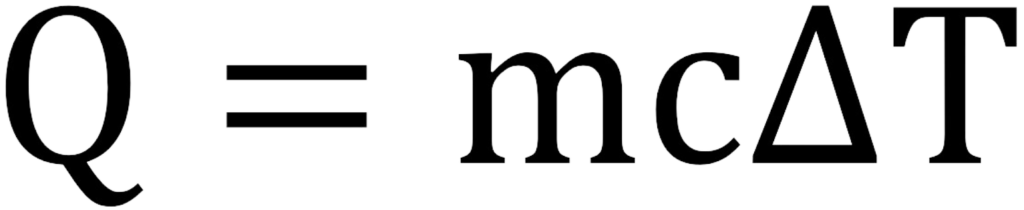
Examples of Thermal Energy:
- A hot cup of coffee: When you touch a hot cup of coffee, you feel warm. This is due to the thermal energy of the liquid present inside the cup, which is transferred to your hand when you touch it.

- Sunlight: The sun emits vast amounts of thermal energy, which warms the Earth and all the other planets near it.
If you want to learn more about it, please click here: The Complete Guide to Thermodynamics.
Chapter 3
Chemical Energy
We have already learned that whenever a chemical reaction takes place, old bonds are broken and new bonds are formed. There will always energy released or absorbed during this process, and the this energy is known as chemical energy.
Chemical energy depends on:
1. The type of chemical bonds present
2. The amount of substance involved
Examples of Chemical Energy:
- Batteries: They always undergo a chemical reaction which results in the release or absorption of energy

- Food: Contains chemical energy that our bodies convert into kinetic and thermal energy for movement and maintaining body temperature.

To know more about chemical energy and understand it better, please click here: Chemical Energy Explained.
Chapter 4
Gravitational Potential Energy
The energy associated with an object due to its position is known as gravitational potential energy (GPE).

The value of Gravitational Potential Energy (GPE) depends on:
1. Mass (m) : more the mass, more will be GPE
2. Height (h) : higher the height, more will be GPE
The formula for gravitational potential energy is:
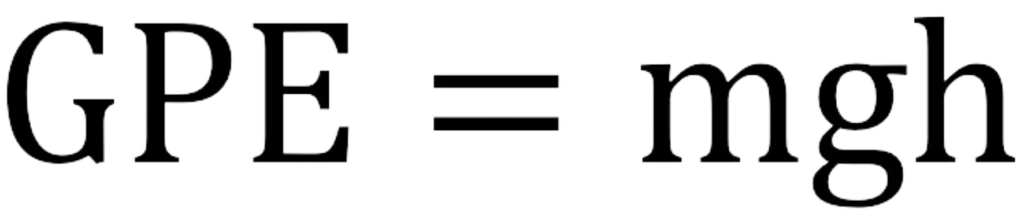
Therefore, the greater the mass and the height, the higher the GPE.

Solved Example : Calculate the gravitational potential energy of a 40 kg boy who is at a height of 20 meters above the ground.
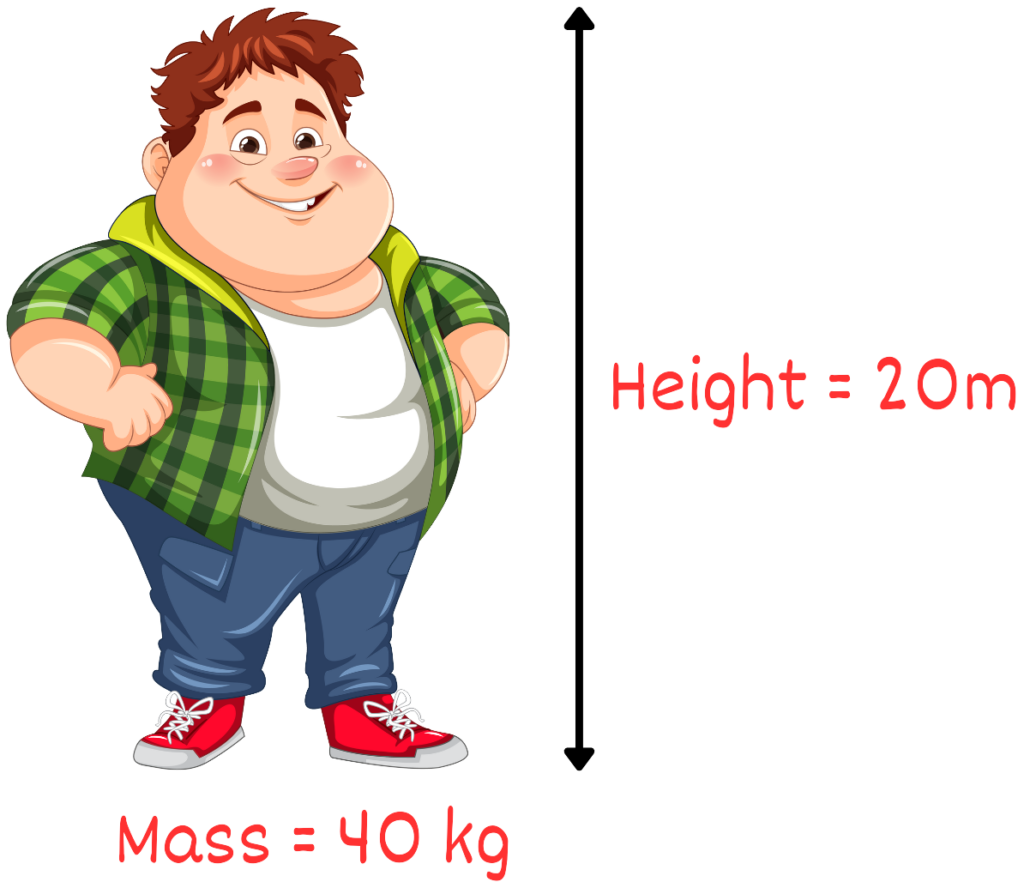
Solution:
Given:
- Mass, m=40kg
- Height, h=20m
- Gravitational acceleration, g=9.81m/s2
Using the formula:
GPE = mgh
= 40 × 9.81 × 20
= 7,848J
Therefore, the gravitational potential energy of the boy is 7,848 joules (J).
Chapter 5
Elastic Potential Energy
Whenever we stretch or compress an object like a spring or rubber band, we apply some energy in doing so. This energy gets stored in that object in the form of elastic potential energy (EPE) and is released when the object returns to its original length.

The amount of elastic potential energy that can be stored in an object depends on two things:
1. Stiffness of the object: The stiffer the object, the more elastic energy it can store.
2. The amount of extension or deformation: The greater the deformation, the more elastic energy is stored.
To know more about elastic potential energy and its examples, as well as the type of energy it is, please click on this link: Elastic Potential Energy Examples.
Chapter 6
Electrical Energy
All the lights and appliances in your home are powered by a special form of energy known as electrical energy. This energy is responsible for the movement of charges inside a wire or any metal, making current flow.

Electrical energy depends on two things:
1. Charge flowing: The more charge that flows, the more electrical energy there is.
2. Potential difference through which the charge flows.
To know more about electrical energy and its formula, click on this link: The Complete Guide to Electrical Energy.
Chapter 7
Magnetic Potential Energy
What is magnetic potential energy?
Whenever we bring an iron object near a magnet, it gets attracted to the magnet. Or whenever we bring two magnets together, they attract or repel each other. In short, this results in motion.
The energy responsible for this motion is known as magnetic potential energy.
Magnetic Potential Energy is also known as “magnetization energy.”
Chapter 8
Nuclear Energy
Almost every one of us has heard or seen this equation: E = mc2. This is the famous equation given by Einstein that explains how mass gets converted into energy.

This equation forms the basis for understanding the energy released in nuclear reactors or nuclear bombs.

Whenever we talk about energy released by fission or fusion of atoms, we refer to nuclear energy.