Qualitative Analysis - GCSE Chemistry
Introduction
- Qualitative analysis is used in chemistry to identify unknown ions by observing their specific colours, reactions, or precipitates.
- Each ion must have a unique test because if two ions gave the same result, it would be impossible to know which one is present.
- In this blog, we are going to study different tests, which are useful in water testing, environmental studies, medical labs, and industry.
What is a Flame Test?
- This is a simple method used in chemistry to identify metal ions based on the colour they produce when heated in a flame.
- When metal ions are heated, their electrons absorb energy and move to higher energy levels.
- As the electrons return to their original levels, they release energy in the form of visible light.
- The colour of the flame depends on the type of metal ion present.
How it is Done:
- A clean wire loop made of platinum or nichrome is dipped into the sample solution.
- It is then held in the blue part of a Bunsen burner flame.
- The flame colour is then carefully observed to see which metal ion is present.
Common Flame Colours:
- Lithium (Li⁺): Crimson red
- Sodium (Na⁺): Bright yellow
- Potassium (K⁺): Lilac
- Calcium (Ca²⁺): Orange-red
- Copper (Cu²⁺): Green

How do we test for Cations?
- Compounds that contain transition metals often have distinct colours. When two chemicals are reacted together and a new solid form that does not dissolve in the solution, this is called a precipitation reaction.
- In solutions, compounds can dissociate into ions, and the positive ions are called cations.
How it is Done:
- A dilute solution of sodium hydroxide (NaOH) can be used to test for certain metal ions.
- It can also help identify ammonium ions by producing characteristic reactions when added to the solution.
Common Results:

How do we test for Anions?
- Anions are negatively charged ions, such as carbonates (CO₃²⁻), sulfates (SO₄²⁻), and halides (Cl⁻, Br⁻, I⁻).
- Each has a specific chemical test:
1. Carbonate Ions (CO₃²⁻):
- To test for carbonate ions, add a few drops of dilute acid such as hydrochloric acid to the sample.
- If effervescence is seen, it shows that carbon dioxide gas is being released.
- To confirm this, the gas is passed through limewater, which turns cloudy or milky, proving the gas is carbon dioxide and confirming the presence of carbonate ions.
2. Sulfate Ions (SO₄²⁻):
- To test for sulfate ions, add dilute hydrochloric acid followed by a few drops of barium chloride solution (BaCl₂).
- If sulfate ions are present, a white insoluble precipitate of barium sulfate (BaSO₄) will form.
- The acid is added first to remove any carbonate ions that could give a false white precipitate.
- The formation of this white solid confirms that sulfate ions are present in the solution.
3. Halide Ions (Cl⁻, Br⁻, I⁻):
- To test for halide ions, first add dilute nitric acid to the sample, then add a few drops of silver nitrate solution (AgNO₃).
- Depending on the halide present, different coloured insoluble precipitates will form: white for chloride (AgCl), cream for bromide (AgBr), and yellow for iodide (AgI).
- The nitric acid helps remove carbonate ions that might interfere with the result, and the colour of the precipitate confirms which halide ion is present.

How does a Flame Photometer Work?
- A Flame photometer is an instrumental method used to identify and measure metal ions in a solution.
How it is worked:
- A sample is heated in a flame, and the light it emits is passed through a spectroscope, producing a spectrum — a pattern of coloured lines.
- Each element gives off light at specific wavelengths, creating a unique spectrum like a fingerprint.
- This helps scientists identify metal ions accurately, even in mixtures where one metal’s colour might hide another in a normal flame test.
Determining Concentrations:
- A Flame photometer can measure the light intensity for solutions with different known concentrations of a metal ion.
- These readings are used to create a calibration curve.
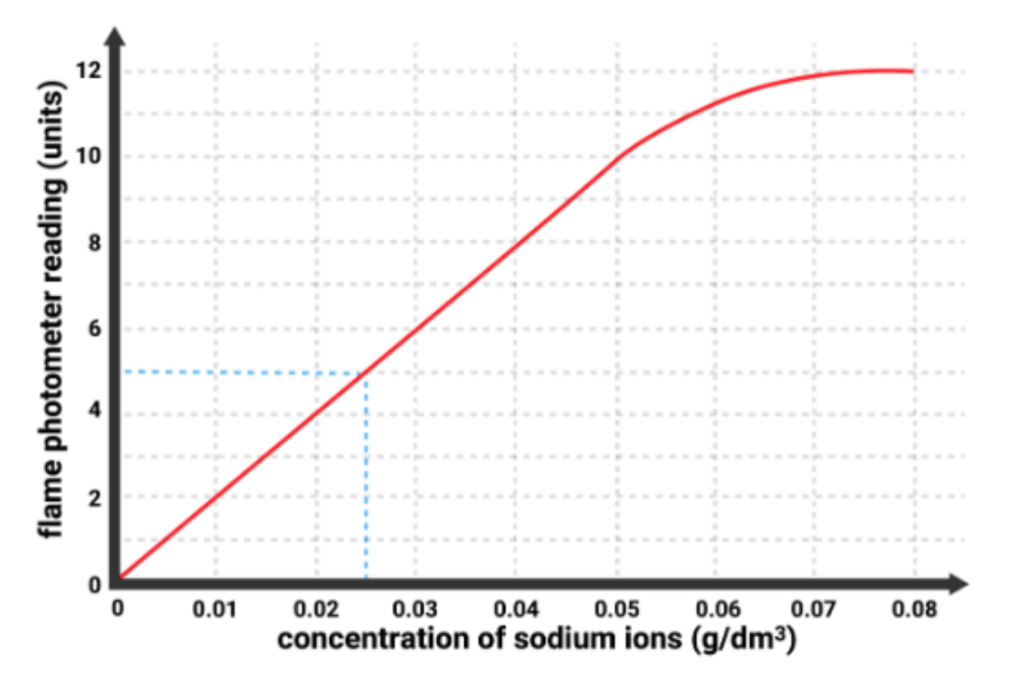
- Once the curve is made, scientists can easily determine the concentration of an unknown sample by comparing its reading to the graph.
- Example: If a solution of sodium ions gave a reading of 5 units on the flame photometer, then the calibration curve allows us to read off that the sample had a concentration of 0.025 g/dm3.
Frequently Asked Questions
Solution:
A flame test is a method used to identify metal ions by the colour they produce in a flame.
Solution:
Because electrons in metal ions absorb energy, move to a higher level, and release energy as light when they return — each metal emits specific wavelengths.
Solution:
Common ones include lithium (red), sodium (yellow), potassium (lilac), calcium (orange-red), and copper (green).
Solution:
Cations (positively charged ions) are tested using sodium hydroxide (NaOH) or ammonia (NH₃) to form coloured precipitates.
Solution:
Yes — Cu²⁺ + NaOH → blue precipitate (copper hydroxide) or Fe³⁺ + NaOH → brown precipitate (iron hydroxide).
Solution:
Anions (negatively charged ions) are tested using specific chemical reactions:
- Carbonates → fizz with acid
- Sulfates → white precipitate with barium chloride
- Halides → coloured precipitate with silver nitrate
Solution:
Because they are fast, accurate, and can detect small amounts of substances better than simple chemical tests.
Solution:
Examples include flame photometry, spectroscopy, chromatography, and mass spectrometry
Solution:
A sample is sprayed into a flame, emitting light. The light is split into a spectrum, and the intensity shows the concentration of metal ions.
Solution:
Because it can measure the amount of metal ions, separate colours in a mixture, and give a unique spectrum for each element — even in mixtures.

