Separating and Purifying Substances - GCSE Chemistry
Introduction
- In chemistry, many materials exist as mixtures.
- To use them properly, scientists separate and purify them using physical properties such as boiling point, solubility, or particle size. These methods keep the chemical composition the same.
- Separation helps obtain pure substances and is widely used in laboratories, industries, and everyday tasks like purifying water or refining fuels.
What are Pure Substances and Mixtures?
- In our daily lives, we often talk about things being “pure,” like pure water or pure juice.
- In chemistry, “pure” means it is made up of only one kind of particle, with nothing else mixed in.
- To understand materials better, we need to know what makes a substance pure and how it differs from a mixture.
Pure Substances:
- A pure substance contains only one type of element or compound.
- It has a fixed composition and specific physical properties such as a sharp melting and boiling point.
- This means every part of the substance is the same.
Example:
- Pure oxygen (O₂), pure water (H₂O), and pure sodium chloride (NaCl).

Mixtures:
- A mixture is made of two or more substances that are not chemically joined.
- Each substance keeps its own properties and can be separated by physical methods like filtration, distillation, or evaporation.
- Mixtures do not have fixed compositions, and their melting or boiling points change over a range of temperatures.
Example:
What are The Main Techniques Used to Separate and Purify Mixtures?
- In chemistry, different methods of separation and purification are used to obtain pure substances from mixtures.
- Each technique is chosen based on the type of mixture and the properties of the materials involved.
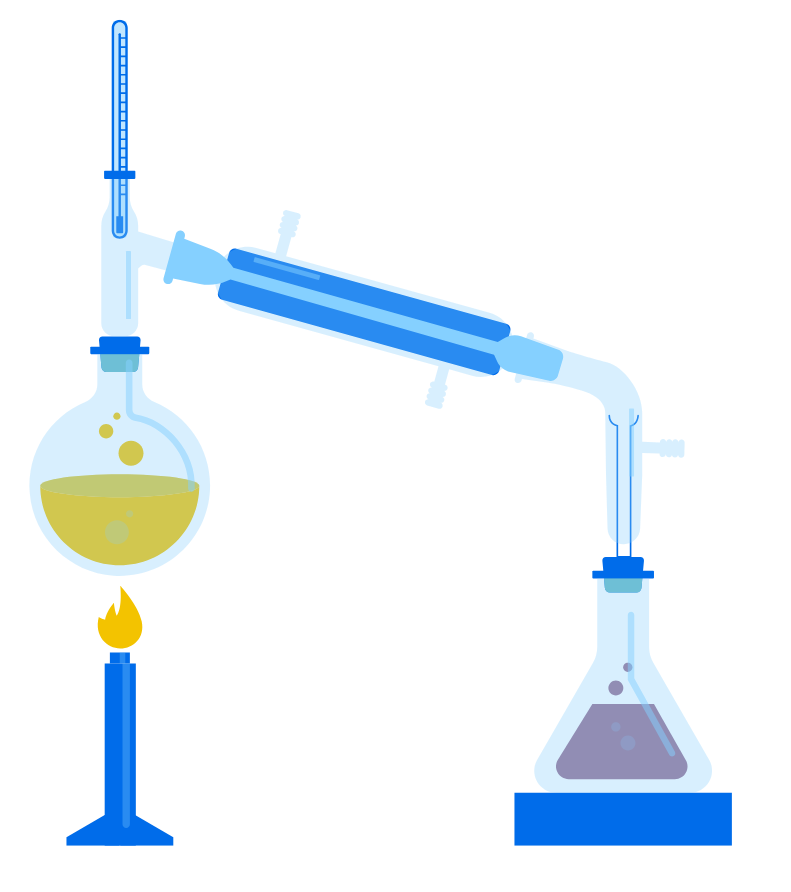
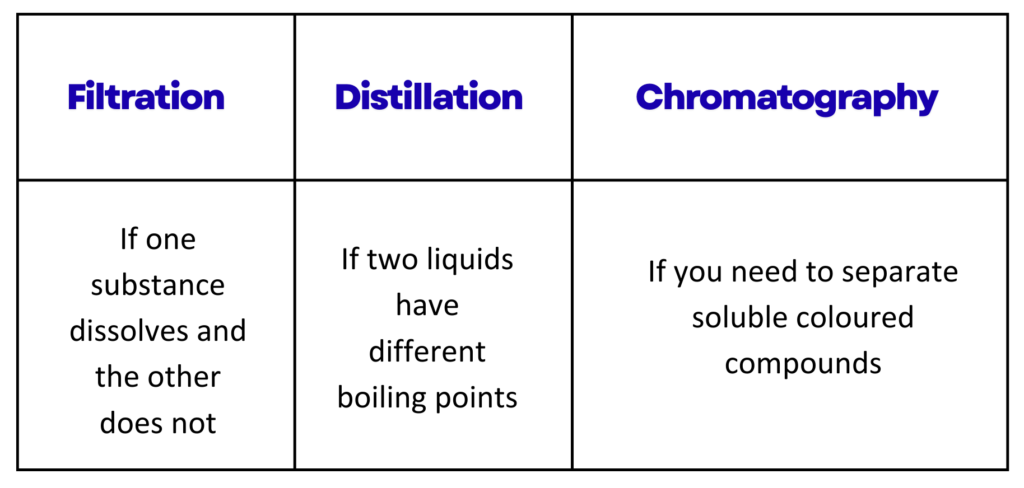
1. Simple Distillation:
- This method is used to separate a solvent from a solution.
- During the process, the solvent is heated until it evaporates.
- The vapour then cools and condenses into a pure liquid, leaving the solute behind.
- It is useful when the components have very different boiling points.
Example:
- Separating pure water from salt water or distilling alcohol for laboratory use.
2. Fractional Distillation
- This method separates liquids with different boiling points.
- The mixture is heated so the liquid with the lowest boiling point evaporates first.
- The vapour passes through a fractionating column and then condenses back into liquid.
- It is useful for separating ethanol and water or crude oil into fuels.
3. Filtration
- This method is used to separate an insoluble solid from a liquid.
- The mixture is poured through filter paper placed in a funnel.
- The solid remains on the paper as residue, while the liquid passes through as filtrate.
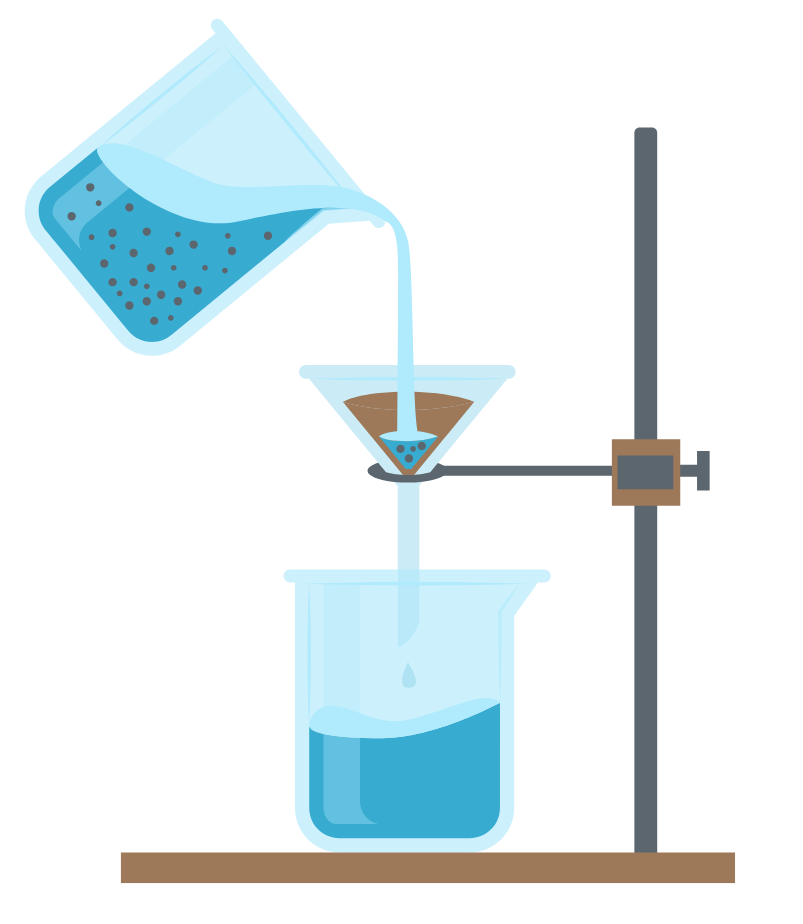
- It is useful for separating substances like sand from water or mud from muddy water.
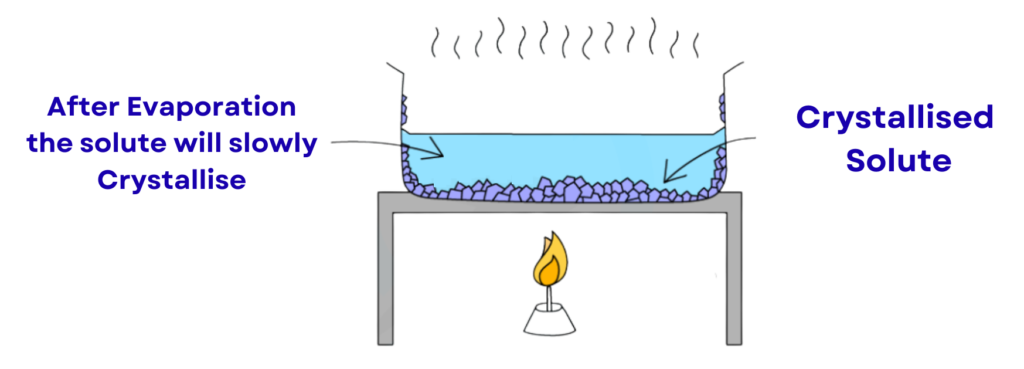
4. Crystallisation:
- This method is used to obtain pure solid crystals from a solution.
- The solution is gently heated until it becomes saturated, then allowed to cool.
- As it cools, the solute forms pure crystals, which can be collected and dried.
- It is useful for producing copper sulfate crystals or salt from sea water.
5. Paper Chromatography:
- This method is used to separate soluble coloured substances such as inks or dyes.
- A small spot of the mixture is placed on chromatography paper, and the paper is set in a solvent.
- As the solvent moves up the paper, the colours spread and separate based on how well they dissolve and travel with the solvent.
- It is useful for identifying different dyes in inks or checking for artificial colours in foods.

How to Choose a Separation Method?
- The choice of separation technique depends on the physical properties of the substances in the mixture, such as boiling point, solubility, and particle size.
Example:
- Choosing the right method is important to achieve complete and accurate separation without losing any substance or altering its chemical composition.
How Can Water be Made Potable and Safe for Use in Chemistry?
- Potable water means water that is safe to drink, though it is not completely pure.
- It has very low levels of microbes and dissolved substances.
1. Treating Waste and Ground Water:
- Water from natural sources contains impurities. To make it potable, it goes through several steps:
- Sedimentation: Large particles settle at the bottom.
- Filtration: Water passes through sand to remove smaller solids.
- Chlorination: Chlorine is added to kill bacteria and germs.
2. Making Sea Water Potable:
- Sea water is purified by distillation. It is filtered, then boiled so pure water vapour forms and condenses, leaving the salt behind.
3. Water for Chemical Analys:
- In laboratories, water used for experiments must be completely pure, either distilled or deionised. This type of water has no dissolved salts or impurities, ensuring that test results are accurate and not affected by unwanted chemical reactions.
Frequently Asked Questions
Solution:
- A pure substance contains only one type of element or compound. It has a fixed composition and definite melting and boiling points.
- Examples include pure water (H₂O) or oxygen gas (O₂).
Solution:
- A mixture contains two or more substances not chemically joined together.
- Each substance keeps its own properties and can be separated by physical methods such as filtration or distillation.
- Examples include air, sea water, and sand with salt.
Solution:
- A pure substance melts and boils at specific temperatures.
- A mixture melts or boils over a range of temperatures because it contains more than one substance.
- For example, pure water boils at 100°C, but salt water boils at a slightly higher temperature.
Solution:
- Simple distillation separates a solvent from a solution.
- It works because the solvent evaporates first, then condenses into pure liquid.
- Example: separating pure water from salt water.
Solution:
- Fractional distillation separates a mixture of liquids that have different boiling points.
- It is used to separate ethanol from water or to separate crude oil into different fuels like petrol and diesel.
Solution:
- Filtration separates insoluble solids from liquids using filter paper.
- Crystallisation is used to obtain pure crystals of a soluble solid by evaporating and cooling the solution.
- Example: forming copper sulfate crystals from solution.
Solution:
- Paper chromatography separates mixtures of soluble substances, especially coloured ones like dyes.
- The solvent moves up the paper, carrying substances at different speeds.
- A pure substance forms one spot, while a mixture forms several.
- The Rf value helps identify each substance.
Solution:
- The practical investigates the composition of inks using simple distillation and paper chromatography.
- Distillation separates the solvent, while chromatography separates the dyes in the ink to show which colours are present.

