Fuel Cells – GCSE Chemistry
Introduction
- A device used to convert Chemical energy into electrical energy is called a cell.
- A fuel cell is a special type of cell that works through electrochemical reactions, usually using hydrogen as fuel and oxygen as the oxidant.
Applications:
What is a Fuel Cell?
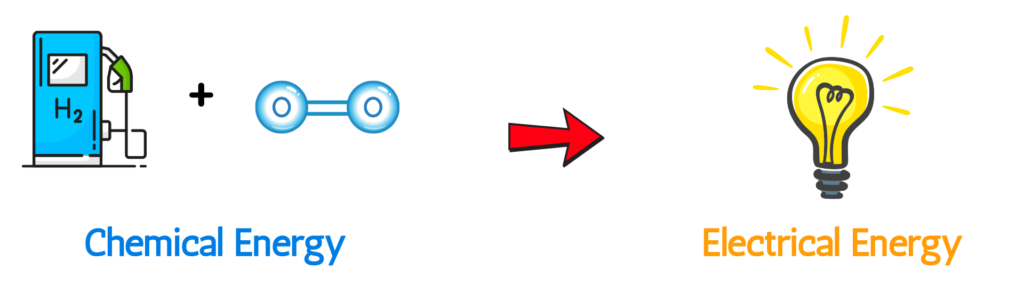
- A fuel cell is a device where fuel (such as hydrogen) reacts with oxygen to directly convert chemical energy into electrical energy, with water as the main by-product.
- Unlike ordinary batteries that store a limited amount of chemicals, fuel cells continuously generate electricity as long as they are supplied with fuel and oxygen.
Fuel cells are particularly important because:
- They produce clean energy with only water as waste.
- They reduce air pollution and greenhouse gases.
- They are more efficient than burning fuels.
- Used in cars and buses as eco-friendly fuel.
- They support a sustainable energy future using hydrogen from renewables.
What is the Structure of a Fuel Cell?
- A practical fuel cell is made by stacking many small cells together, and each cell has several important layers:
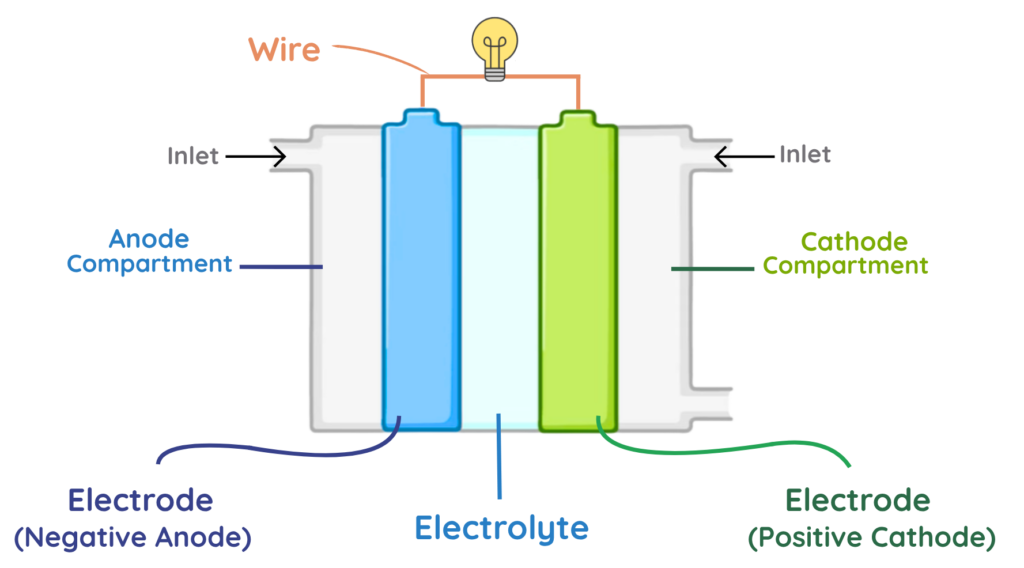
Electrolyte
- The electrolyte is the middle layer that allows ions to pass but blocks electrons, forcing them to move through the wire to create current.
- In alkaline fuel cells,
Potassium Hydroxide (KOH)
is commonly used as the electrolyte.
Electrodes
- There are two electrodes: the anode (negative) and the cathode (positive).
- At the anode, hydrogen is supplied and split into protons and electrons.
- At the cathode, oxygen reacts with these protons and electrons to form water.
Compartments
- The anode compartment carries hydrogen to the anode, while the cathode compartment carries oxygen to the cathode.
- These spaces ensure gases reach the right place for reaction.
Inlets
- Inlets are openings for gases to enter the cell.
- Hydrogen enters at the anode side and oxygen at the cathode side.
Wire (External Circuit)
- The wire connects the anode and cathode outside the cell.
- Electrons travel through this wire, producing electric current to power devices.
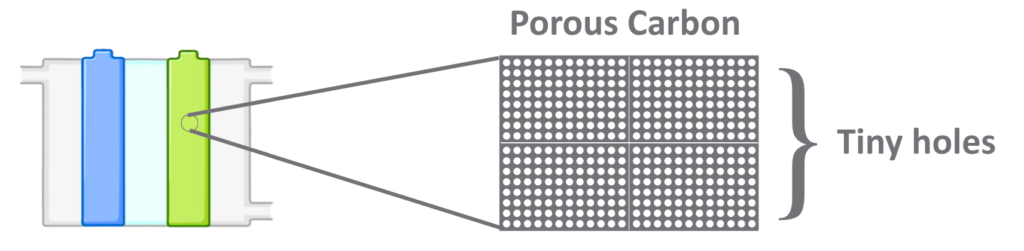
Tiny Holes in Electrodes
- The electrodes are porous (full of tiny holes).
- These holes spread gases evenly and also let water pass out, preventing blockage.
How Does a Fuel Cell Work?
- The fuel cell works by using hydrogen and oxygen to produce electricity, with water as the only by-product.
- Its working can be understood in the following steps:
Hydrogen supply at the anode –
- Hydrogen gas enters the cell from the fuel inlet and reaches the anode.
- Here, with the help of a catalyst, each hydrogen molecule splits into protons (H⁺ ions) and electrons (e⁻).
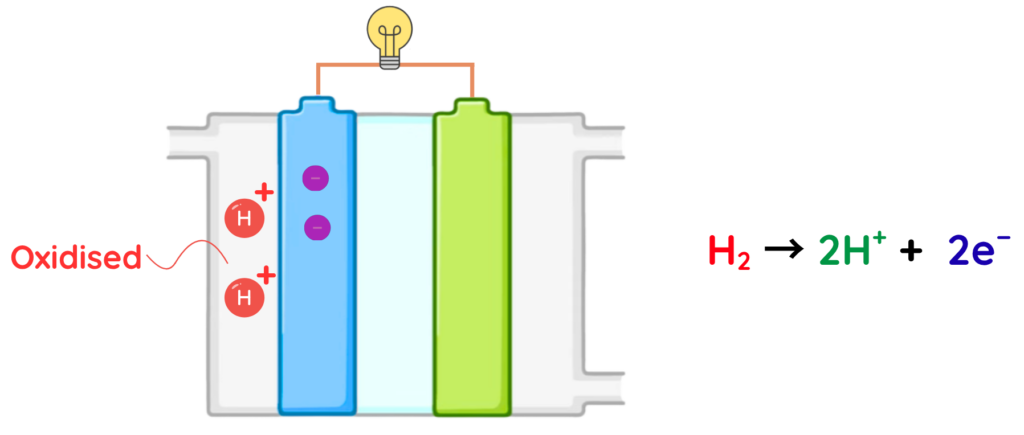
Movement of protons and electrons –
- The protons (H⁺) pass through the electrolyte (commonly KOH or a proton-exchange membrane) to reach the cathode.
- The electrons (e⁻) cannot cross the electrolyte, so they travel through the external wire, creating an electric current that can power devices.
Oxygen supply at the cathode –
- Oxygen gas enters from the oxidant inlet and reaches the cathode.
- Here, oxygen molecules combine with the incoming protons (H⁺) and the returning electrons (e⁻) from the wire.
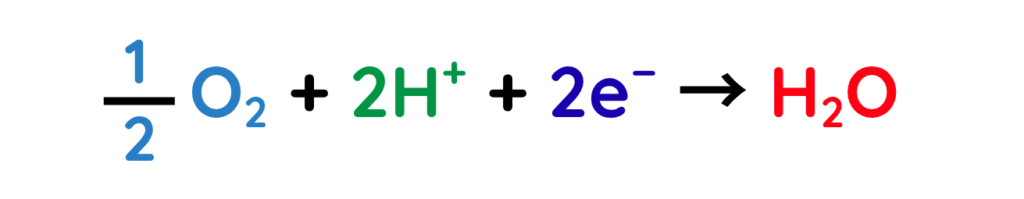
Formation of water and removal –
- The product formed at the cathode is water, which comes out as a by-product through the outlet.
- Any excess water is removed from the bottom of the fuel cell to keep the system running smoothly.
Continuous Process –
- As long as hydrogen and oxygen are supplied, this process continues, providing a steady flow of electricity and heat.
Chemical Reactions and Equations in a Fuel Cell
- In a fuel cell, the reactions occur in two parts: half-reactions at each electrode, and then the overall cell reaction.
At the Anode (oxidation of hydrogen):

- Hydrogen molecules split into protons (which move through the electrolyte) and electrons (which travel through the external circuit).
At the Cathode (reduction of oxygen):
- Oxygen from the inlet reacts with the protons and electrons to form water.

Overall Cell Reaction:
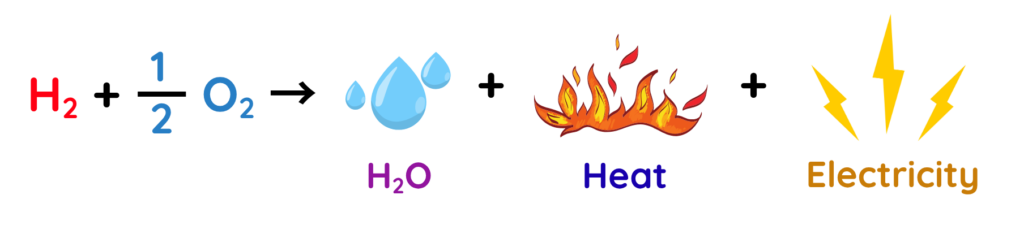
Pros and Cons of Fuel Cells
Pros of Fuel Cells
- Only need hydrogen and oxygen, which are abundant.
- Do not produce carbon dioxide or pollutants as waste.

- Produce water as the only by-product.
- More efficient than combustion engines.
- Simple design can last longer than batteries.
- Less polluting to dispose of compared to batteries.
- Operates quietly without noise.
- Can provide continuous power as long as fuel is supplied.
- Can be used in homes, vehicles, industries, and space missions.
Cons of Fuel Cells
- Hydrogen is a gas and needs more space to store than fossil fuels or batteries.
- Storage and transport of hydrogen is dangerous and costly.
- Hydrogen is explosive when mixed with air.
- Making hydrogen fuel itself requires energy.
- Fuel cells are expensive due to costly materials like platinum.
- Need special infrastructure for refueling stations.
- Limited durability, can degrade over time.
- If hydrogen is made from fossil fuels, it can still cause pollution indirectly.
Frequently Asked Questions
Solution:
A fuel cell is a device that converts chemical energy from a fuel (like hydrogen) into electrical energy through a chemical reaction with oxygen.
Solution:
Unlike batteries, fuel cells do not run out or need recharging — they produce electricity continuously as long as fuel and oxygen are supplied.
Solution:
A fuel cell has two electrodes (anode and cathode) and an electrolyte between them that allows ions to move while keeping gases separate.
Solution:
At the anode, hydrogen gas is split into protons and electrons.
Solution:
At the cathode, oxygen reacts with protons and electrons to form water.
Solution:
2H₂ + O₂ → 2H₂O + energy (electricity + heat)
Solution:
They are efficient, produce clean energy, and the only by-product is water — making them environmentally friendly.
Solution:
They are expensive to make, require pure hydrogen (hard to store), and the production of hydrogen may release carbon emissions.

