Heat Transfer Unveiled A Comprehensive Guide to Thermal Energy
Welcome back to MyExamRevision! In today’s blog post, we’re diving into the fascinating world of heat transfer. When objects are heated, energy flows and is stored in their thermal energy reserves. Let’s explore the three methods through which heat can be transferred and the unique ways they impact different materials and environments.


If you’re looking for a tailored and comprehensive approach to understanding heat transfer dynamics – conduction, convection, and radiation – your search ends here. My personalized tutoring is designed to cater to your unique learning style, pace, and academic goals. Together, we’ll unravel the complexities of energy movement and excel in grasping this fundamental concept.
Whether you’re preparing for your upcoming studies or seeking to deepen your knowledge, take the next step by clicking this link: Physics Private Tutoring
Unlocking the Mysteries of Gravity, Weight, and Energy delves deep into the fascinating realm of physics, offering insights into fundamental principles. Explore this captivating topic now: Unlocking the Mysteries of Gravity, Weight, and Energy
Contents
Chapter 1
Conduction: Vibrational Energy Transmission
Let’s start our exploration with conduction, a heat transfer mechanism that primarily occurs in solids. The underlying principle here revolves around the vibrating particles within a substance, which transfers energy to their neighbouring particles through these vibrations.

Imagine heating one end of a metal rod. As the energy is supplied, it’s absorbed by the particles at that end, intensifying their kinetic energy and causing them to vibrate more vigorously. These high-energy particles then collide with adjacent particles, transmitting their energy in a chain reaction. This transfer of kinetic energy continues throughout the substance until a state of equilibrium is achieved, resulting in uniform temperature distribution.

Chapter 2
Thermal Conductivity: Efficiency of Conduction
The efficiency of conduction is quantified by a property known as thermal conductivity. Metals, characterized by their closely packed atomic arrangements, exhibit high thermal conductivity.

Consequently, metals can rapidly transfer heat energy over relatively large distances. This property is harnessed in various applications, from cooking utensils to heat sinks in electronics.
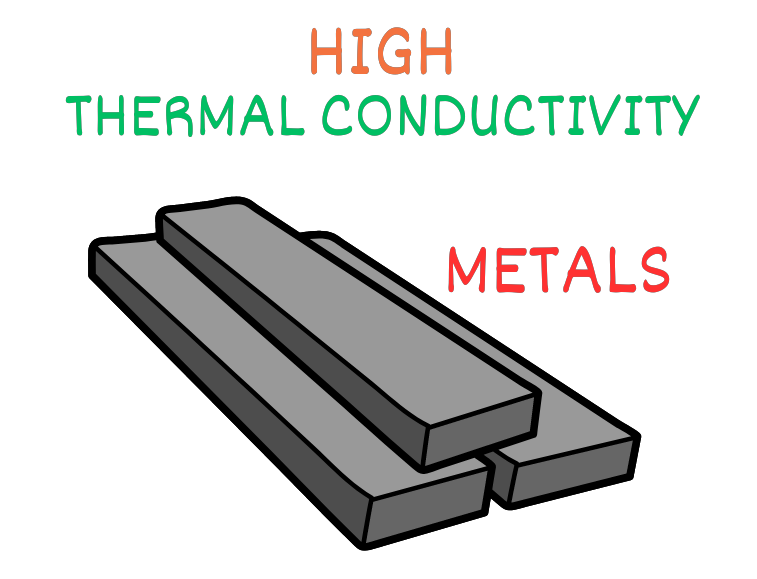
On the other hand, substances with lower thermal conductivity, such as plastics, serve as effective insulators. This is why plastic handles on cooking utensils stay cooler even when the metal part is heated. The ability to control heat transfer by exploiting thermal conductivity variations is essential in engineering and design.

Chapter 3
Convection: Fluid Dynamics of Heat Transfer
Convection takes center stage when we shift our focus to fluids, encompassing both liquids and gases. Unlike solids, the particles in fluids are not fixed in place; they can move freely.

When a fluid is heated, its particles gain kinetic energy, leading to increased motion and random diffusion. This phenomenon causes the more energetic particles to move away from the heat source and towards cooler regions.

As the warmer fluid rises due to its reduced density, it creates an upward flow. Conversely, the cooler, denser fluid near the heat source descends. This circulation of fluid due to temperature differences is referred to as a convection current. You might have witnessed convection currents in action when observing a pot of water heating on a stove. The warmer water near the bottom rises, while cooler water descends to take its place. This movement contributes to the even distribution of temperature within the fluid.
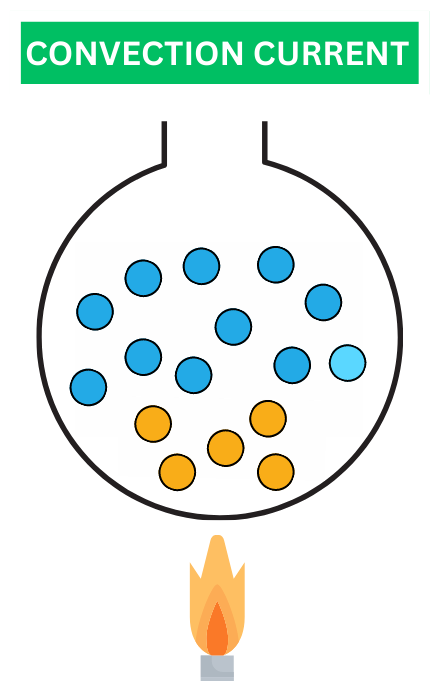
Convection currents are not limited to kitchen scenarios – they play a crucial role in various natural phenomena. For instance, ocean currents are driven by convection currents, which in turn influence climate patterns around the world.

Chapter 4
Radiation: The Silent Traveler of Heat
While conduction and convection rely on particles for heat transfer, radiation offers an entirely different mechanism. In contrast to the first two methods, radiation doesn’t require a medium or particles to propagate. It can occur even in the vacuum of space. This makes radiation a vital player in the universe’s energy balance.

Radiation occurs through electromagnetic waves, specifically in the infrared region of the electromagnetic spectrum. All objects with a temperature above absolute zero emit radiation. The amount of radiation emitted by an object is directly related to its temperature – hotter objects emit more radiation.

You may have experienced this phenomenon firsthand when standing near a barbecue grill. Even if you’re not touching the grill, you can feel the heat radiating from it.

Radiation is an integral aspect of Earth’s energy budget. The Sun’s energy, radiated as electromagnetic waves, reaches our planet and is absorbed by the surface. The surface then re-emits this energy as lower-energy infrared radiation, contributing to Earth’s overall heat balance.
Chapter 5
Conclusion: Unraveling the Tapestry of Heat Transfer
And there you have it – a comprehensive journey through the intricate world of heat transfer. From the vibrating particles in solids to the fluid dynamics of convection and the silent journey of radiation, these mechanisms shape our understanding of energy exchange. Each method, governed by unique principles, offers insights into the behaviour of heat energy in various scenarios
As you go about your daily life, remember that the transfer of heat energy is at the heart of countless natural processes, technological innovations, and environmental phenomena. We hope this exploration has deepened your appreciation for the multifaceted nature of heat transfer. Until our next adventure, stay curious and keep exploring the wonders of the physical world!
Gain deeper insights into the world of Heat Transfer: Conduction, Convection, and Radiation. Explore further by clicking the link: Difference between Conduction Convection and Radiation




