Earth and Atmospheric Science – GCSE Chemistry
Introduction
- Earth and Atmospheric Sciences is the study of the Earth’s surface, interior, and the atmosphere around it.
- It helps us understand natural events like earthquakes, weather, and climate change.
This field brings together many topics such as:
- Why weather changes every day
- How the climate is changing over time
- What effects humans have on nature

Moral Duty of Humans:
- So, it becomes one of our moral duty to take care of the environment we are living in. We can better understand natural events and find ways to protect the planet. It connects science with real-life problems like pollution, global warming, and natural disasters.
Basics of Earth’s Atmosphere And The Gases Contained In It
Earth’s Atmosphere:
- The Earth’s atmosphere is a layer of gases that surrounds our planet and makes life possible.
- It protects us from harmful sun rays, keeps the Earth warm, and allows us to breathe.
- It is made up of nitrogen (78%), oxygen (21%), and small amounts of other gases.
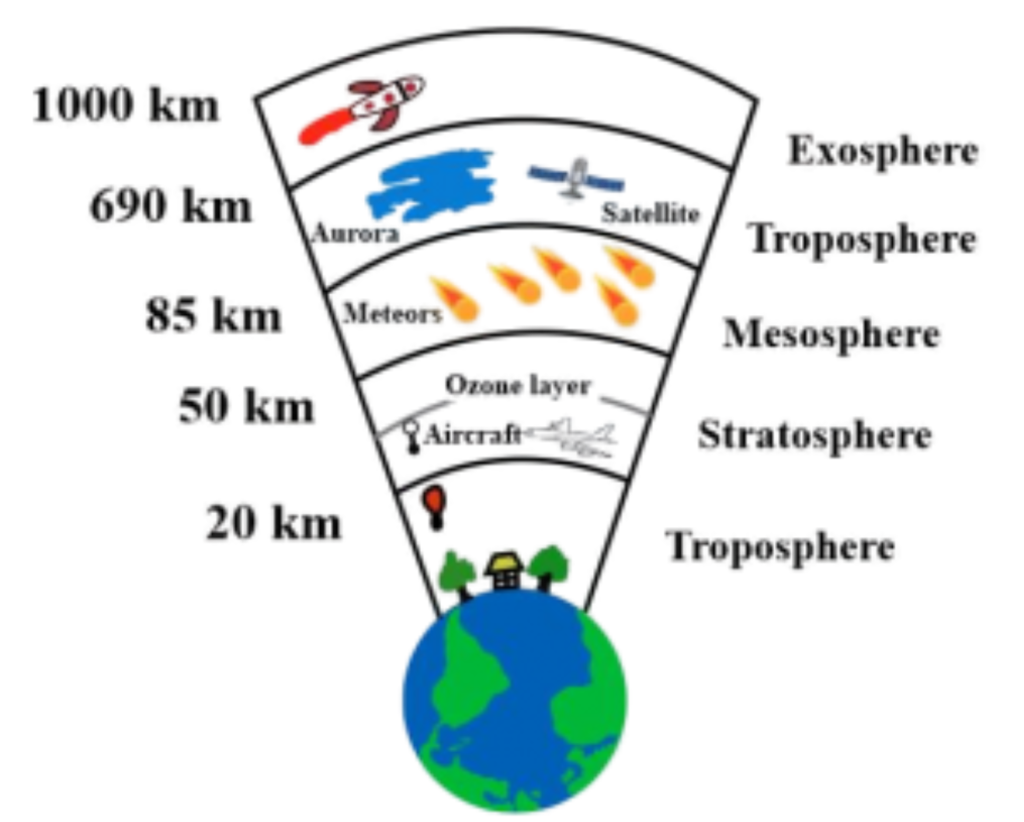
The atmosphere has five main layers:
- Troposphere – where weather happens
- Stratosphere – contains the ozone layer
- Mesosphere – burns up meteors
- Thermosphere – has the auroras
- Exosphere – the outermost layer, merging into space
Diagrammatically, it can be represented as:
Gases contained in Earth’s Atmosphere:
- Earth’s atmosphere is made up of a mixture of gases that surround the planet and support life. These gases play vital roles in breathing, weather, and protecting Earth from harmful radiation.
Here are the main gases present in the atmosphere:
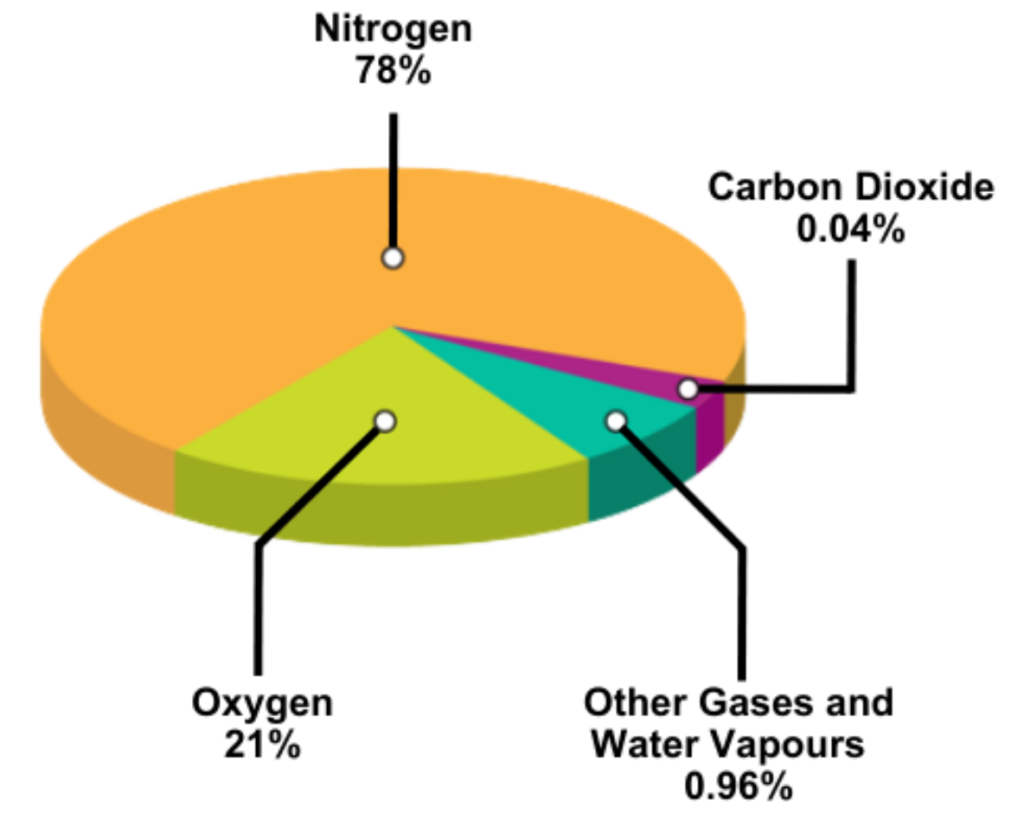
Nitrogen (N₂) – 78%
- The most abundant gas
- Helps in plant growth through the nitrogen cycle
Oxygen (O₂) – 21%
- Essential for breathing and combustion
- Supports life on Earth
Argon (Ar) – 0.93%
- An inert gas
- Does not react easily with other elements Carbon
Dioxide (CO₂) – 0.04%
- Important for photosynthesis in plants
- A greenhouse gas that affects Earth’s temperature
Other gases (less than 0.03%)
- Include neon, helium, methane, krypton, hydrogen, and water vapor
- Though small in amount, they influence weather, temperature, and radiation
Condensation of Water Vapours
- Condensation is the process where water vapour (gas) in the air changes into liquid water. This happens when warm, moist air cools down. As the air temperature drops, it can’t hold as much moisture, so the excess water forms droplets.
How Condensation Happens:
- Step #1: Evaporation occurs when water from oceans, lakes, and other sources heats up and turns into water vapour.
- Step #2: As this warm, moist air rises, it cools at higher altitudes.

- Step #3: When it cools enough to reach the dew point, water vapour condenses onto small particles in the air such as dust or pollen.
- Step #4: This results in the formation of tiny water droplets that group together to form clouds, fog, or dew.
Equation of condensation can be written as:
H₂O (g) → H₂O (l)
Explanation:
- H₂O (g) represents water in the gaseous state (water vapour).
- H₂O (l) represents water in the liquid state.
- The arrow shows that water vapour condenses into liquid water when it cools down.
There’s no new substance formed during condensation — it’s the same water molecules, just changing form from gas to liquid.
Why Condensation Is Important:
- Essential for the Water Cycle: It allows water to return to Earth’s surface from the atmosphere.
- Controls Earth’s Temperature: Through cloud formation, it helps regulate the planet’s heat balance.
Everyday Examples of Condensation:
- Water droplets forming on the outside of a cold glass.
- Bathroom mirrors fogging up after a hot shower.
- Mist forming on car windows during winter.
Important Note:
- As the Earth cooled, water vapour condensed and formed oceans. Carbon dioxide (CO₂) from the atmosphere dissolved into these oceans. Some of the CO₂ reacted with water to form carbonic acid, and later formed carbonates, which got stored in rocks and shells. This process reduced the amount of CO₂ in the atmosphere, helping to cool the planet.
PHOTOSYNTHESIS
- Photosynthesis is the process by which green plants, algae, and certain bacteria convert light energy from the sun into chemical energy in the form of glucose (a type of sugar).
- This process occurs mainly in the leaves of plants and is essential for sustaining life on Earth.
Where It Happens:
- Photosynthesis takes place inside plant cells, in special structures called chloroplasts.
- These contain a green pigment called chlorophyll, which absorbs sunlight. Chlorophyll gives plants their green color and plays a crucial role in capturing solar energy.
Raw Materials Required:
- Sunlight – The energy source
- Carbon Dioxide (CO₂) – Taken from the air through tiny leaf openings called stomata
- Water (H₂O) – Absorbed from the soil by plant roots
The Word Equation:
Carbon dioxide + Water + Light energy → Glucose + Oxygen
The Balanced Chemical Equation:
6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
- 6CO₂ = six molecules of carbon dioxide
- 6H₂O = six molecules of water
- C₆H₁₂O₆ = glucose (sugar used as plant food)
- 6O₂ = six molecules of oxygen (released into the air)
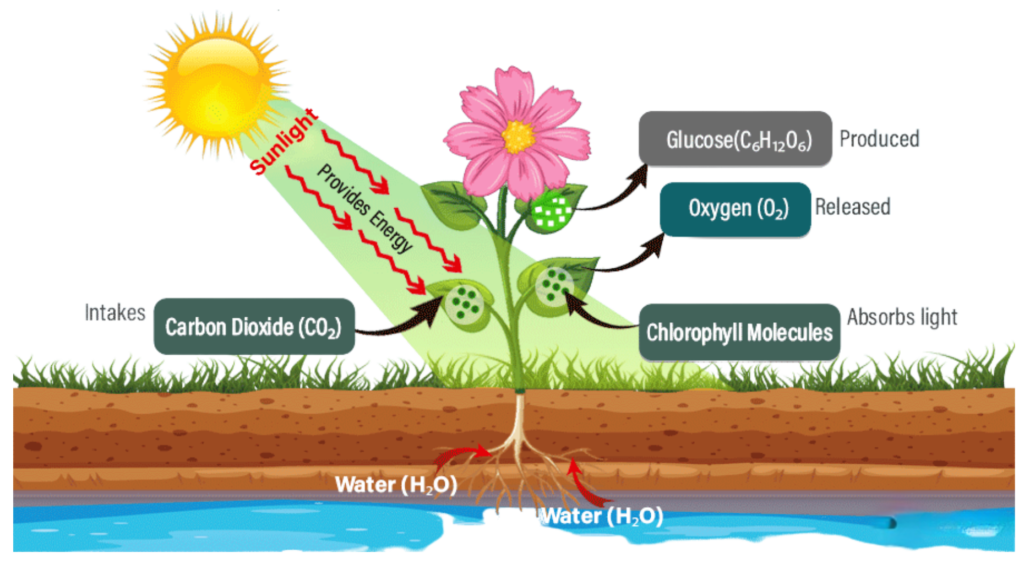
Diagrammatically, Photosynthesis can be shown as above.
Why Photosynthesis Is So Important
Why Photosynthesis Is So Important:
1. Food Production:
- It produces glucose, which plants use for energy and growth.
- This sugar also supports animals that eat plants — directly or indirectly.
2. Oxygen Release:
- Oxygen is a by-product of photosynthesis and is released into the air.
- All animals, including humans, need oxygen to survive.
3. Carbon Dioxide Removal:
- Plants absorb CO₂ from the atmosphere, helping reduce the amount of this greenhouse gas and controlling global warming.
4. Foundation of Life:
- It is the base of all food chains on Earth.
- All living organisms either directly or indirectly depend on photosynthesis for energy.
Chemical Test of O2
- In Earth and Atmospheric Sciences, understanding the composition of the atmosphere is important — especially detecting gases like oxygen, which is vital for life and combustion.
- One simple way to test for the presence of oxygen is through the “glowing splint test”.

Observation:
- Oxygen supports combustion, so it makes the glowing splint catch fire again.
- This confirms that the gas being tested contains oxygen.
Greenhouse Effect
The Greenhouse Effect is a natural process that warms the Earth’s surface. It occurs when certain gases in the atmosphere trap heat from the Sun.
- These gases are known as greenhouse gases, and they include carbon dioxide (CO₂), methane (CH₄), nitrous oxide (N₂O), water vapor (H₂O), and ozone (O₃).
How the Greenhouse Effect Works:
- Sunlight reaches Earth and passes through the atmosphere.
- Some of the energy is absorbed by the Earth’s surface, warming it.
- The Earth then re-emits this energy as heat (infrared radiation).
- Greenhouse gases in the atmosphere absorb and trap some of this heat.
- This trapped heat is radiated back toward the Earth’s surface, keeping it warm.
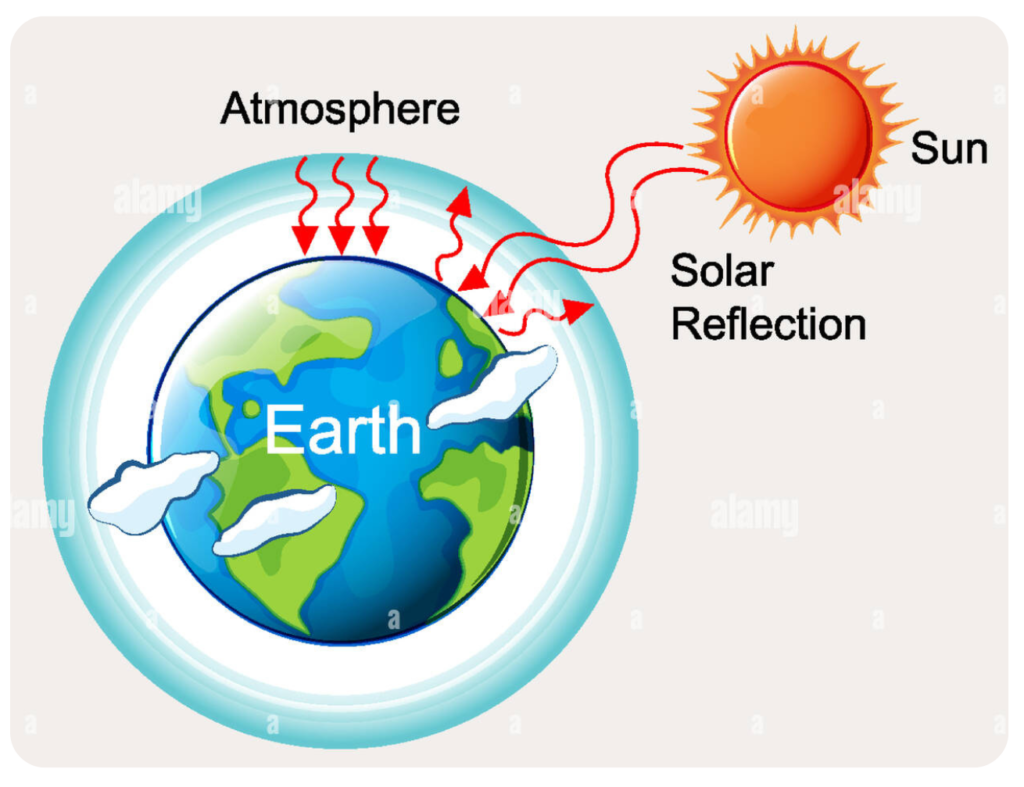
Why the Greenhouse Effect Is Important:
- It keeps Earth’s average temperature around 15°C (59°F).
- Without it, the planet would be too cold for most life to exist (around -18°C).
- It helps maintain a stable climate system.
Environment Exploitation
Environment Exploitation:
- Environmental exploitation refers to the overuse or misuse of natural resources by humans for economic or personal gain. This includes actions that harm nature without allowing it time to recover, leading to long-term damage to the Earth’s ecosystems.
Forms of Environmental Exploitation:
1. Deforestation:
- Cutting down forests for urban development, leading to loss of biodiversity and climate imbalance.
2. Industrial Pollution:
- Releasing toxic chemicals into air, water, and soil through factories and vehicles.
3. Overuse of Water Resources:
- Drawing excessive water for farming or cities, reducing river flows and drying up lakes.
4. Soil Degradation:
- Intensive farming and improper land use leading to soil erosion and loss of fertility.
Consequences of Environmental Exploitation:
- Climate change due to increased greenhouse gas emissions
- Loss of biodiversity and extinction of species
- Polluted air and water, affecting human and animal health
- Resource scarcity, like clean water, fresh air, and fertile land.
Conclusion:
- While nature provides us with everything we need to survive, unchecked exploitation can lead to irreversible damage. To prevent this, we must promote sustainable practices, use resources wisely, and care for our planet.
Frequently Asked Questions
Solution:
The atmosphere provides oxygen to breathe, protects us from harmful solar radiation, keeps the planet warm through the greenhouse effect, and allows weather systems to form.
Solution:
They are gases like carbon dioxide (CO₂), methane (CH₄), and water vapor that trap heat in the Earth’s atmosphere. While they are natural, too much of them leads to global warming.
Solution:
Most green plants perform photosynthesis. However, some plants like parasitic plants or fungi do not photosynthesize and rely on other organisms for food.
Solution:
Chlorophyll is the green pigment in plants that absorbs light energy from the sun and helps convert it into chemical energy during photosynthesis.
Solution:
Temperature (cooling promotes condensation) Humidity (more moisture increases the chance) Surface conditions (smooth, cold surfaces attract more condensation)
Solution:
In Earth’s early history, intense volcanic eruptions released large amounts of gases into the air. These gases slowly built up the first atmosphere, which was very different from today’s air.

