Chemical energy is a form of _____ energy?
If you are looking for a one work answer:
“Chemical Energy is a form of Potential Energy”
Chemical Energy, when you hear this word for the first time, what is the thing that comes to your mind? Normally many people associate chemicals with only something like acids or bases.
But that is not true.

Everything around us is made of chemicals. It is the food you eat, a wooden table or even your very own body, everything is made of chemicals and everything has chemical energy inside it.

In this particular article, we will discuss the following things:
1) What is Actually Chemical Energy?
2) How is Chemical Energy a form of Potential Energy?
3)Some common applications of Chemical Energy.
So lets begin, but before that if you want to have a basic understanding of all the different stores of energy, please click on this link: Stores of Energy: Types, Transfer, and Applications
Contents
Chapter 1
What is Actually Chemical Energy?
Everything around us is made of matter, which is nothing but atoms and molecules. If we go deeper into the Chemistry of this, we know that atoms react with one another during chemical reactions to form bonds which can be ionic bonds or covalent bonds.
So, in any type of chemical reaction, new bonds are formed and old bonds are broken. And whenever there is a bond formed or broken, there will Energy that will be released or absorbed.
This Energy that is released or absorbed during a chemical reaction is known as “Chemical Energy”
So the only thing necessary for a change in Chemical Energy is a Chemical reaction.
To understand this better, remember some basic processes like:
Photosynthesis,
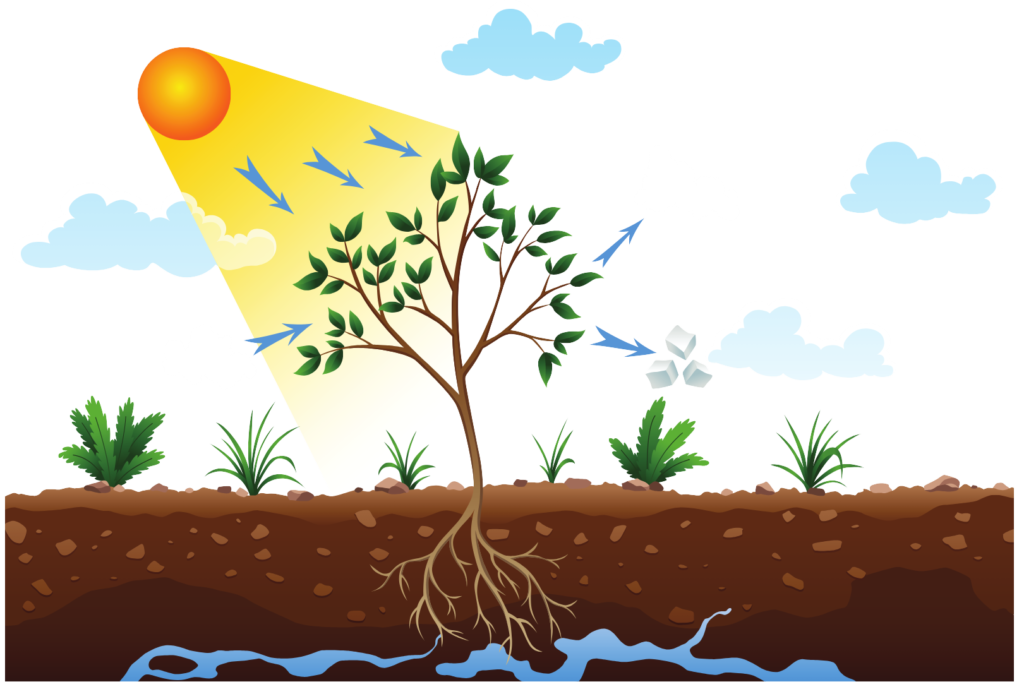
Burning of glucose in your body,
The burning of fuel in your car,

Rusting of iron

These all are nothing but chemical reactions, so they all are accompanied by change in Chemical Energy. In some of these, chemical energy is absorbed and in some chemical energy is released.
When you consume food, your body breaks down these chemical bonds, releasing the energy stored within, which your cells use to function.
Similarly, when fuel burns in an engine, the chemical bonds in the fuel are broken, releasing energy that moves your car.
These reactions can either release energy, which can be used to do work, or they absorb energy from the surroundings.
Chapter 2
How is Chemical Energy a Form of Potential Energy?
Chemical energy is categorized as potential energy because it is the energy stored inside the bonds of a compound, waiting to be released.
This stored energy is due to the position and arrangement of atoms within molecules, very similar to how a stretched rubber band has Elastic potential energy (link) due to its stretched state.

1. Energy Stored: Chemical bonds hold atoms together in molecules. These bonds can be thought of as tiny springs connecting the atoms. Energy will be required to break these bonds later in the future and we can think of them as energy stored.
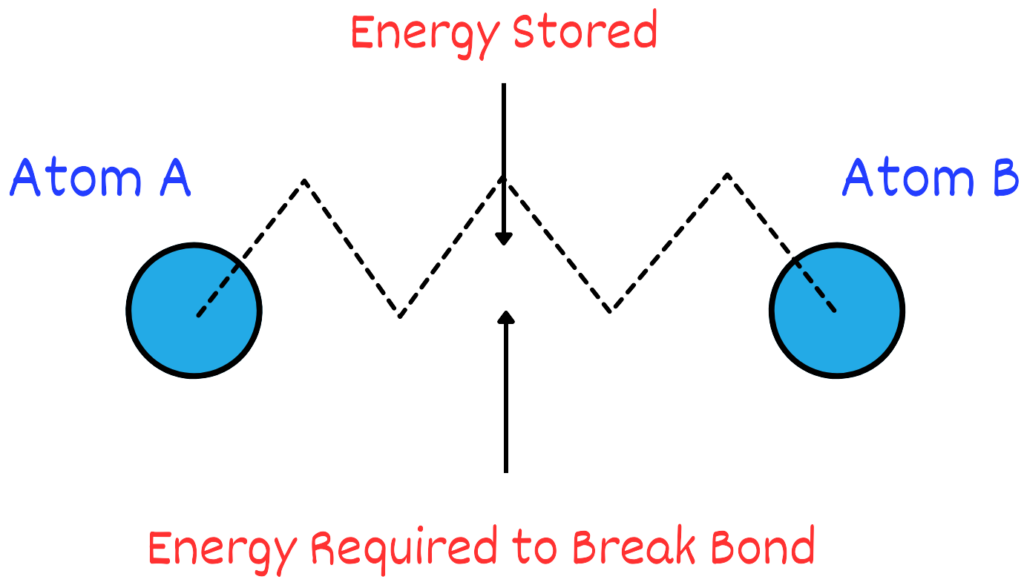
2. Energy Released: When chemical bonds are broken, such as during digestion or combustion, the stored potential energy is converted into other forms of energy, like kinetic energy (movement) or thermal energy (heat). This transformation from stored energy to usable energy is a hallmark of potential energy.
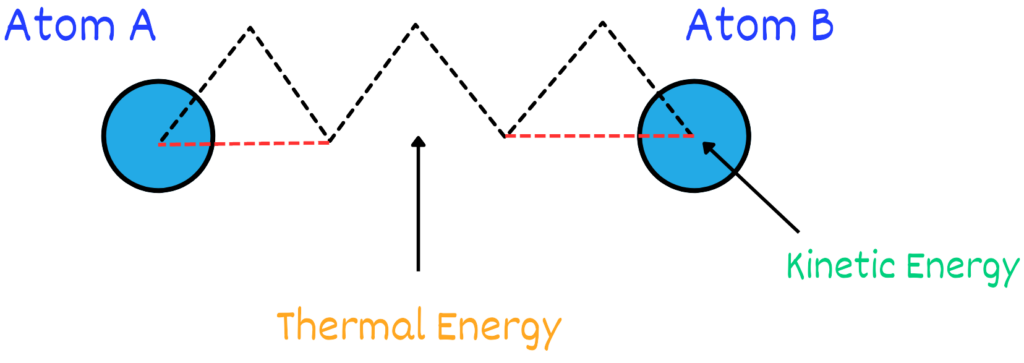
In summary, we can say that chemical energy is a type of potential energy because whenever a chemical reaction takes place and results in the formation of new bonds and molecules. This energy is stored in these molecules which can later be used for different processes.
Chapter 3
Common Applications of Chemical Energy

1. Biological Processes:
- Photosynthesis: Plants convert light energy into chemical energy stored in glucose through photosynthesis. In this process, new bonds are formed when glucose is formed

- Respiration: When we breathe, the cells in our body convert the chemical energy in food into ATP (which is a form of energy for our body), The reaction breaks down the bonds of glucose present in our food.
2. Combustion:
- Fuels: Gasoline, diesel, and natural gas are rich in chemical energy. When burned in engines or power plants, the chemical bonds in these fuels break, releasing energy that is converted into mechanical work or electricity.
3. Batteries:
- Electrochemical Cells: Batteries store chemical energy and convert it into electrical energy to power various devices, from smartphones to electric vehicles.

Chapter 4
Conclusion
Chemical energy is a form of potential energy. Understanding how chemical energy is stored, released, and utilized helps us in the basic steps behind many natural and man-made processes.

Whether in the bonds of the food we eat, the fuel that drives our vehicles, or the batteries that power our gadgets, chemical energy is all around us, silently enabling the many activities.
For a deeper understanding of different energy stores, their types, transfers, and applications, explore our comprehensive guide: Stores of Energy: Types, Transfer, and Applications.